Cardiac arrhythmia: CPVT in molecular detail
Calcium ions play crucial roles in our bodies: they shape part of the cardiac action potential, mediate contraction of both cardiac and skeletal muscle, allow for the release of neurotransmitter and hormones, and regulate gene transcription, cell motility and much more. When normal Ca2+ entry is impaired, these can result in severe genetic diseases that are often fatal. Ca2+ enters the cytoplasm through specialized channels, selective for Ca2+. Our lab is dedicated to understanding how these channels work in both native and diseased states.
Our main focus is on two types of Ca2+ selective channels: voltage-gated calcium channels (CaVs) and ryanodine receptors (RyRs). Both CaVs and RyRs are dynamic proteins that can change their state depending on various input signals. It is impossible to fully appreciate their intricate functioning without knowing the 3D structure. On the other hand, having a 3D structure alone is not sufficient, because they can adopt multiple conformations that affect the passage of ions . We use protein crystallography to obtain atomic models, thus generating hypotheses about their function. We use electrophysiology and biochemical techniques to test these hypotheses.
Ryanodine Receptors
RyRs are channels that release Ca2+ from the sarcoplasmic/endoplasmic reticulum. In cardiac muscle, the activation of CaVs evokes an increase in cytoplasmic Ca2+ concentration. The RyR detects the increase and releases more Ca2+ into the cytoplasm, thus ‘enforcing’ the signal. In skeletal muscle, CaVs and RyRs are thought to communicate directly through protein-protein interactions.
Mutations in RyRs are linked to several genetic diseases. Mutations in the skeletal muscle isoform (RyR1) can lead to central core disease and malignant hyperthermia, one of the major causes of death due to use of halogenated anaesthetics. Mutations in the cardiac isoform (RyR2) may result in catecholamineric polymorphic ventricular tachycardia (CPVT) and arrhythmogenic right ventrical dysplasia type 2 (ARVD2), two devastating conditions that may lead to cardiac arrhythmias and result in sudden cardiac death.
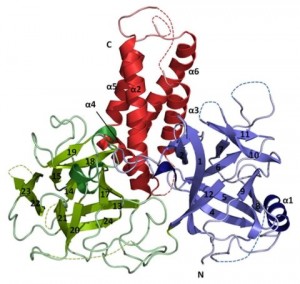
RyRs are the largest ion channels currently known (with sizes up to 2.2MDa), built up by 4 identical subunits. They are regulated by a wide array of small molecules (such as caffeine) and auxiliary proteins (calmodulin, FKBP, kinases, phosphatases,…). We currently know very little about the RyR atomic structure. We recently determined the crystal structure of a disease ‘hot spot’, covering ~11% of the entire protein. Our major focus is to generate more crystal structures of important domains and their interactions with auxiliary proteins.
Mutations in RyRs are linked to several genetic diseases. Mutations in the skeletal muscle isoform (RyR1) can lead to central core disease (CCD) and malignant hyperthermia (MH), one of the major causes of death due to use of halogenated anaesthetics. Mutations in the cardiac isoform (RyR2) may result in catecholamineric polymorphic ventricular tachycardia (CPVT) and arrhythmogenic right ventrical dysplasia type 2 (ARVD2), two devastating conditions that may lead to cardiac arrhythmias and result in sudden cardiac death.
RyRs are the largest ion channels currently known (with sizes up to 2.2MDa), built up by 4 identical subunits. They are regulated by a wide array of small molecules (such as caffeine) and auxiliary proteins (calmodulin, FKBP, kinases, phosphatases,…). We currently know very little about the RyR atomic structure. We recently determined the crystal structure of a disease ‘hot spot’, covering ~11% of the entire protein. Our major focus is to generate more crystal structures of important domains and their interactions with auxiliary proteins.
Voltage-gated Calcium Channels
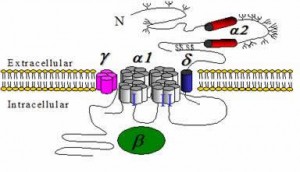
CaVs are able to detect differences in voltage across the plasma membrane: when an excitable cell depolarizes to a sufficient level, they open and allow Ca2+ to enter. CaVs thus convert an electrical signal into an intracellular chemical signal. Their dysfunction leads to various severe and often lethal genetic diseases, and they form the targets for many drugs that are being used to treat cardiovascular diseases, hypertension, epilepsy, and chronic pain. Despite their importance, we still lack a profound insight into how CaVs work. This is due, in part, to the limited amount of high-resolution data describing their precise architecture.
CaVs can be built up by multiple subunits. The CaVα1 subunit allows passage of the ions. The other subunits (β, γ, α2δ) help in trafficking of the channel and regulate its kinetic properties. In addition, a myriad of auxiliary proteins (kinases, phosphatases, calmodulin, scaffolding molecules,…) can interact with CaVs in a dynamic manner, regulating the channel properties. CaVs are sensitive integrators: the output (the time and amount of Ca2+ entering the cell) is dependent on several input signals (Ca2+ concentration, phosphorylation status, various protein-protein interactions). Our primary focus lies with dissecting the mechanisms whereby auxiliary proteins regulate the channels.
Techniques
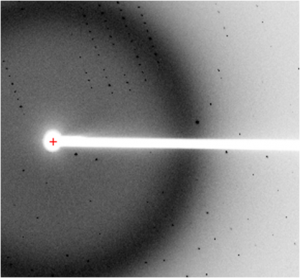
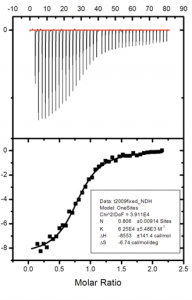
We use a variety of complementary biochemical and biophysical techniques to investigate ion channels. Examples include x-ray crystallography to elucidate the 3D structure of individual subunits, domains, and their complexes with ligands, isothermal titration calorimetry (ITC) and surface plasmon resonance (SPR) to detect and quantify protein-ligand and protein-protein interactions, circular dichroism (CD) to analyze secondary structure and protein stability, and electrophysiology (two-electrode voltage clamp and Planar lipid bilayer electrophysiology) to measure the ionic conductance and gating properties.